Bohr model of first 20 elements – The Bohr model of the first 20 elements embarks us on a captivating journey into the realm of atomic structure. Niels Bohr’s pioneering model, introduced in 1913, laid the groundwork for our understanding of the arrangement and behavior of electrons within atoms.
This model, despite its limitations, remains a cornerstone in our comprehension of atomic physics. It provides a framework for visualizing the energy levels and electron configurations of elements, paving the way for advancements in chemistry, physics, and materials science.
Introduction to Bohr Model
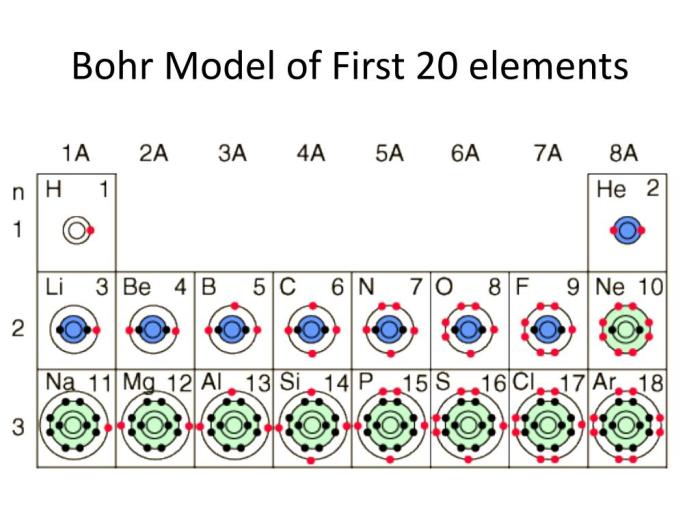
The Bohr model, proposed by Niels Bohr in 1913, was a revolutionary step in the understanding of atomic structure. Prior to Bohr’s model, scientists had no clear understanding of how atoms were structured or how they behaved.
The Bohr model introduced several key principles and assumptions:
Basic Principles
- Electrons orbit the nucleus in fixed, circular paths called energy levels.
- Each energy level has a specific energy associated with it.
- Electrons can only exist in specific energy levels, and they cannot exist between levels.
- When an electron moves from a higher energy level to a lower energy level, it releases a photon of light with a frequency that corresponds to the energy difference between the two levels.
- When an electron absorbs a photon of light with a frequency that corresponds to the energy difference between two energy levels, it moves from a lower energy level to a higher energy level.
Structure of the Bohr Model
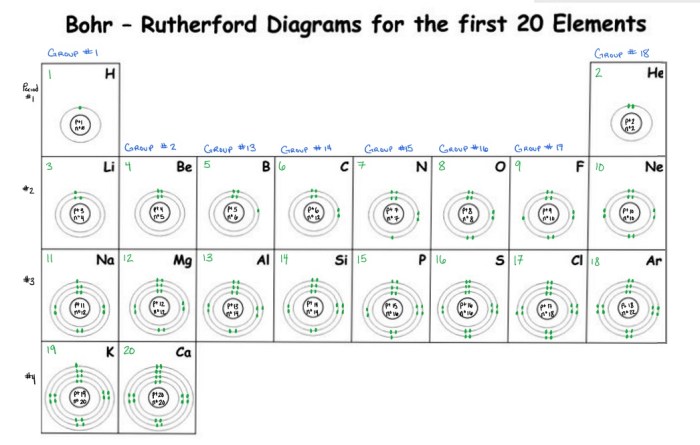
The Bohr model, proposed by Niels Bohr in 1913, describes the structure of an atom. It introduces the concept of energy levels and electron distribution within those levels.
Energy Levels and Orbitals
In the Bohr model, electrons occupy specific energy levels around the nucleus. These energy levels are quantized, meaning they exist at discrete values. The energy of an electron increases as it moves to higher energy levels.Each energy level is divided into sublevels called orbitals.
Orbitals are regions around the nucleus where electrons are most likely to be found. The shape of an orbital depends on its energy level and sublevel.
The Bohr model of the first 20 elements provides a simplified representation of atomic structure. This model is useful for understanding the basic principles of quantum mechanics. Like the recent US v. Mendenhall case brief , which highlights the importance of legal interpretation, the Bohr model emphasizes the need for a clear understanding of the underlying principles.
By studying the Bohr model, we can gain insights into the structure and behavior of atoms, which are the building blocks of matter.
Electron Distribution
Electrons fill orbitals in a specific order based on their energy. The lowest energy level, called the first energy level, can hold up to two electrons. The second energy level can hold up to eight electrons, and so on.Within each energy level, electrons occupy orbitals in a specific sequence:
1. s orbital
Can hold up to 2 electrons
2. p orbital
Can hold up to 6 electrons
3. d orbital
Can hold up to 10 electrons
4. f orbital
Can hold up to 14 electronsThe electron configuration of an element describes the distribution of its electrons in orbitals. For example, the electron configuration of helium (He) is 1s 2, indicating that it has two electrons in its first energy level’s s orbital.
Energy Level and Electron Configuration Table, Bohr model of first 20 elements
The following table shows the energy levels and electron configurations of the first 20 elements:| Element | Symbol | Atomic Number | Electron Configuration ||—|—|—|—|| Hydrogen | H | 1 | 1s 1|| Helium | He | 2 | 1s 2|| Lithium | Li | 3 | 1s 22s 1|| Beryllium | Be | 4 | 1s 22s 2|| Boron | B | 5 | 1s 22s 22p 1|| Carbon | C | 6 | 1s 22s 22p 2|| Nitrogen | N | 7 | 1s 22s 22p 3|| Oxygen | O | 8 | 1s 22s 22p 4|| Fluorine | F | 9 | 1s 22s 22p 5|| Neon | Ne | 10 | 1s 22s 22p 6|| Sodium | Na | 11 | 1s 22s 22p 63s 1|| Magnesium | Mg | 12 | 1s 22s 22p 63s 2|| Aluminum | Al | 13 | 1s 22s 22p 63s 23p 1|| Silicon | Si | 14 | 1s 22s 22p 63s 23p 2|| Phosphorus | P | 15 | 1s 22s 22p 63s 23p 3|| Sulfur | S | 16 | 1s 22s 22p 63s 23p 4|| Chlorine | Cl | 17 | 1s 22s 22p 63s 23p 5|| Argon | Ar | 18 | 1s 22s 22p 63s 23p 6|| Potassium | K | 19 | 1s 22s 22p 63s 23p 64s 1|| Calcium | Ca | 20 | 1s 22s 22p 63s 23p 64s 2|
Quantum Numbers: Bohr Model Of First 20 Elements
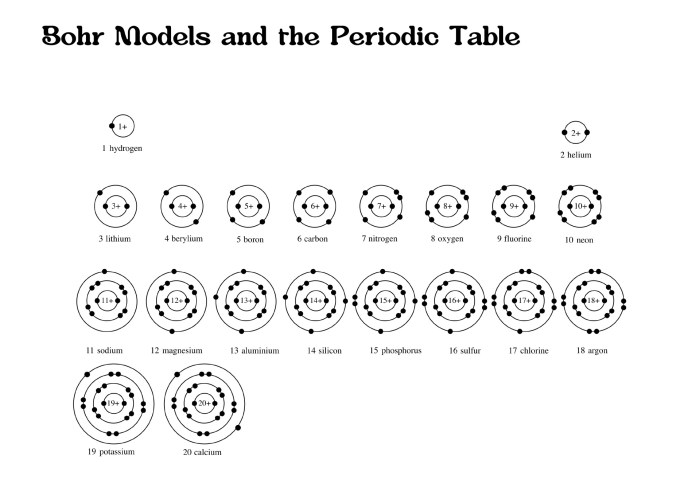
The Bohr model introduced the concept of quantized energy levels for electrons. However, it lacked a detailed explanation of how electrons occupy these levels. Quantum numbers were introduced to address this issue, providing a more comprehensive understanding of electron behavior and the structure of atoms.
Quantum numbers are a set of four numbers that describe the properties of electrons within an atom. They provide information about the electron’s energy, shape, orientation, and spin, allowing for a more accurate description of the electron’s state.
Principal Quantum Number (n)
The principal quantum number (n) describes the energy level of an electron. It is an integer starting from 1, with higher values representing higher energy levels. The principal quantum number determines the size of the electron’s orbital and its distance from the nucleus.
Azimuthal Quantum Number (l)
The azimuthal quantum number (l) describes the shape of an electron’s orbital. It is an integer ranging from 0 to n-1. Different values of l correspond to different orbital shapes, such as s, p, d, and f orbitals.
Magnetic Quantum Number (ml)
The magnetic quantum number (ml) describes the orientation of an electron’s orbital in space. It is an integer ranging from -l to +l. Different values of ml represent different orientations of the orbital around the nucleus.
Spin Quantum Number (ms)
The spin quantum number (ms) describes the intrinsic spin of an electron. It can have two values: +1/2 or -1/2. The spin quantum number indicates the direction of the electron’s spin, either clockwise or counterclockwise.
Quantum numbers play a crucial role in understanding the behavior of electrons in atoms. They allow us to predict the energy levels of electrons, the shapes of their orbitals, and their orientations in space. This information is essential for understanding the chemical properties of elements and the formation of chemical bonds.
Limitations of the Bohr Model
While the Bohr model provided a groundbreaking understanding of the atom, it had certain limitations. It could not fully explain the behavior of electrons in all atoms, particularly in those with more than one electron.
Inability to Explain Certain Atomic Phenomena
- Energy Level Discrepancies:The Bohr model predicted that electrons in different energy levels should have specific, fixed energies. However, experiments showed that the energy levels of electrons in atoms were not always discrete, and they could exist in a range of energies.
- Electron Spin:The Bohr model did not account for the spin of electrons, a fundamental property that affects their behavior.
- Atomic Spectra:The Bohr model could not fully explain the complex patterns observed in the atomic spectra of elements, which contain information about the energy levels and transitions of electrons.
Applications of the Bohr Model
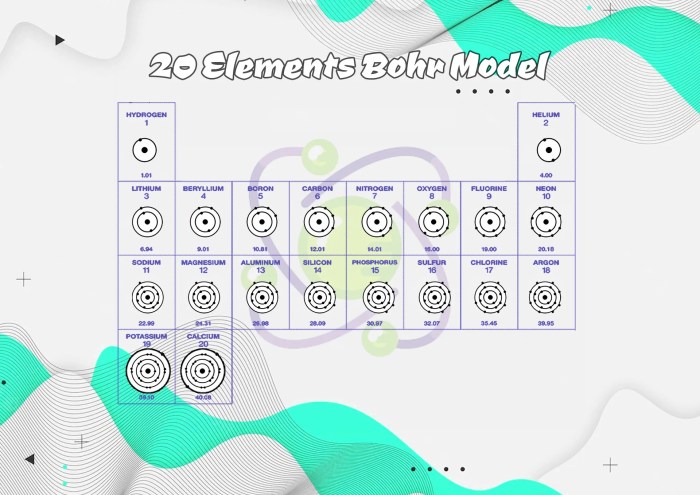
The Bohr model has found numerous applications in understanding atomic structure and behavior, particularly in the early stages of quantum mechanics. It has provided valuable insights into the properties of atoms and their interactions.
Chemistry
- The Bohr model has played a significant role in explaining the periodic trends observed in the chemical properties of elements. By assigning electrons to specific energy levels, the model helps understand the chemical bonding behavior of atoms.
- It aids in predicting the chemical reactivity of elements based on their electronic configurations. For instance, elements with a full outermost energy level are chemically inert, while those with incomplete outermost energy levels are more reactive.
Physics
- The Bohr model has contributed to the development of quantum mechanics by introducing the concept of quantized energy levels. This idea laid the foundation for understanding atomic spectra and the behavior of electrons in atoms.
- It has been used to explain the emission and absorption of light by atoms, forming the basis of atomic spectroscopy. The model helps predict the wavelengths of light emitted or absorbed by atoms during electronic transitions.
Materials Science
- The Bohr model has been instrumental in understanding the electronic properties of materials. By calculating the energy levels of electrons in different materials, scientists can predict their electrical and optical properties.
- It has helped in the development of semiconductor devices, such as transistors and solar cells, by providing insights into the behavior of electrons in semiconductors.
FAQ Resource
What is the significance of the Bohr model?
The Bohr model was a groundbreaking theory that revolutionized our understanding of atomic structure. It introduced the concept of quantized energy levels and explained the arrangement of electrons within atoms.
What are the limitations of the Bohr model?
The Bohr model successfully explained the behavior of hydrogen-like atoms but failed to account for the more complex behavior of atoms with multiple electrons. It also did not explain the wave-particle duality of electrons.
How does the Bohr model contribute to our understanding of chemistry?
The Bohr model provides a framework for understanding the chemical properties of elements. It helps explain why certain elements are more reactive than others and how electrons are involved in chemical reactions.