Elements compounds and mixtures images – Embark on a captivating journey into the realm of chemistry with our comprehensive exploration of elements, compounds, and mixtures. Through stunning images and expert insights, we unravel the intricate relationships and properties that define these fundamental building blocks of matter.
Delve into the unique characteristics of elements, the bonding forces that shape compounds, and the dynamic behavior of mixtures. Discover the practical applications of these substances in everyday life, industry, and scientific research.
Elements, Compounds, and Mixtures
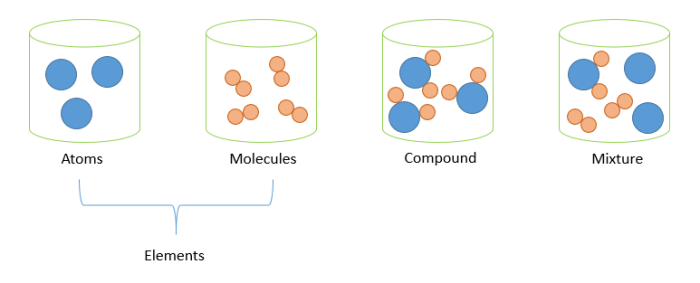
In chemistry, matter can be classified into three distinct categories: elements, compounds, and mixtures.
Elements, Elements compounds and mixtures images
- Elements are the fundamental building blocks of matter.
- They are composed of a single type of atom.
- Examples include hydrogen (H), oxygen (O), and gold (Au).
Compounds
- Compounds are substances composed of two or more different elements chemically bonded together.
- They have a fixed composition and cannot be separated into their constituent elements by physical means.
- Examples include water (H 2O), salt (NaCl), and carbon dioxide (CO 2).
Mixtures
- Mixtures are combinations of two or more elements or compounds that are not chemically bonded together.
- They have a variable composition and can be separated into their constituent components by physical means.
- Examples include air, seawater, and alloys.
| Characteristic | Element | Compound | Mixture |
|---|---|---|---|
| Composition | Single type of atom | Fixed, different elements | Variable, different elements/compounds |
| Bonding | N/A | Chemical | Physical |
| Separation | Not possible | Chemical means | Physical means |
Expert Answers: Elements Compounds And Mixtures Images
What is the difference between an element, a compound, and a mixture?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. A compound is a substance composed of two or more elements chemically combined in fixed proportions. A mixture is a combination of two or more substances that are not chemically combined and retain their individual properties.
How can we separate elements from compounds and mixtures?
Elements can be separated from compounds and mixtures using various techniques such as distillation, chromatography, and electrolysis. Compounds can be separated from mixtures using methods like filtration and crystallization.