Water hardness can affect cleaning by servsafe. This is a topic that has been studied extensively, and there is a wealth of information available on the subject. In this article, we will explore the impact of water hardness on cleaning, and we will provide some tips on how to mitigate the effects of hard water.
Water hardness is a measure of the amount of dissolved minerals in water. The most common minerals that contribute to water hardness are calcium and magnesium. Hard water can cause a number of problems, including scale buildup, soap scum, and decreased cleaning effectiveness.
Water Hardness: Understanding and Impact on Cleaning

Water hardness, a crucial factor in cleaning processes, can significantly affect the effectiveness of cleaning agents and equipment. This article delves into the concept of water hardness, its types, and its impact on cleaning operations. It also explores methods to mitigate water hardness and industry guidelines for cleaning in different water hardness conditions.
Understanding Water Hardness
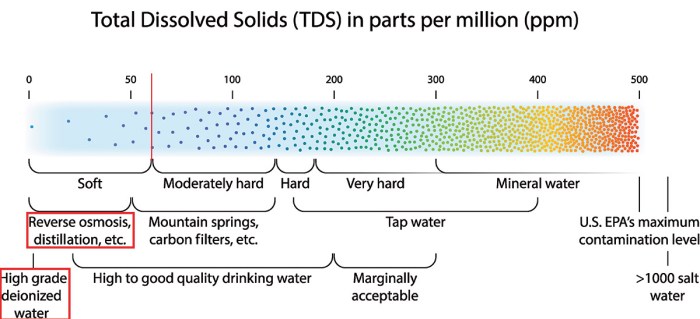
Water hardness refers to the concentration of dissolved minerals, primarily calcium and magnesium ions, in water. It is measured in units of parts per million (ppm) of calcium carbonate (CaCO3). Water hardness is classified into three main types:
- Soft water:Less than 100 ppm CaCO3
- Moderately hard water:100-300 ppm CaCO3
- Hard water:Over 300 ppm CaCO3
Temporary Hardness, Water hardness can affect cleaning by servsafe
Temporary hardness is caused by the presence of calcium and magnesium bicarbonates. These minerals can be removed by boiling water, which converts them into insoluble carbonates that precipitate out of solution.
Permanent Hardness
Permanent hardness is caused by the presence of calcium and magnesium sulfates, chlorides, and nitrates. These minerals cannot be removed by boiling water and require more advanced treatment methods, such as ion exchange or reverse osmosis.
Impact of Water Hardness on Cleaning

Water hardness significantly affects the effectiveness of cleaning agents, particularly detergents and soaps. In hard water, calcium and magnesium ions form insoluble complexes with the cleaning agents, reducing their ability to remove dirt and grime. This can result in:
- Reduced cleaning efficiency
- Increased consumption of cleaning agents
- Formation of scale on surfaces and equipment
Scale Formation
Scale is a hard, mineral deposit that forms on surfaces and equipment when water evaporates, leaving behind dissolved minerals. Scale can clog pipes, reduce water flow, and damage equipment. It is particularly problematic in areas with high water hardness.
Answers to Common Questions: Water Hardness Can Affect Cleaning By Servsafe
What is water hardness?
Water hardness is a measure of the amount of dissolved minerals in water. The most common minerals that contribute to water hardness are calcium and magnesium.
How does water hardness affect cleaning?
Hard water can cause a number of problems, including scale buildup, soap scum, and decreased cleaning effectiveness.
What are some ways to mitigate the effects of hard water?
There are a number of ways to mitigate the effects of hard water, including using water softeners, filtration systems, and adding vinegar or citric acid to the water.